1. Introduction
Grapevine (
Vitis vinifera) is one of the most ancient crops cultivated worldwide for the production of both fresh grapes and wine, which represents an important source of income for many countries. Europe is the leading global wine producer, with about 50% of the world’s vine-growing area, with 3.3 million ha (Food and Agriculture Organization of the United Nations Statistics Division, FAOSTAT, 2018). Grapevine can be affected by virus and virus-like diseases that may significantly reduce the productive life of the plants as well as the grape production in terms of the quantity and quality of the berries [
1]. In fact, the grapevine has been described as the crop that hosts the largest number of plant viruses, with more than 85 viral species known to infect this important crop to date [
2,
3].
Grapevine Roditis leaf discoloration-associated virus (GRLDaV) was identified as a new member of the family
Caulimoviridae, genus
Badnavirus, infecting grapevine in 2015 [
4]. GRLDaV has been associated with a grapevine disease reported in Greece more than thirty years ago, the Roditis leaf discoloration disease (RLD), which produces discolorations and deformations of the leaves, reduction in the number and size of grape berries, and a lower sugar content [
5]. The analysis by high throughput sequencing (HTS) of a grapevine sample showing typical RLD symptoms from a Greek vineyard allowed the identification of GRLDaV and the recovery of its full genome [
4]. The GRLDaV genome consists of a single molecule of circular double-stranded DNA (between 6988 and 7097 nt in length) that includes four open reading frames (ORFs) and a non-coding region. ORFs 1, 2, and 4 encode for hypothetical proteins of unknown function. ORF3 encodes a large polyprotein containing the movement protein (MP), the coat protein (CP), the protease, the reverse transcriptase (RT), and the RNaseH [
4]. GRLDaV has been shown to be mechanically transmissible. Although no biological vectors have been identified to transmit GRLDaV to date, most badnaviruses are spread by different mealybug species [
6].
After its first identification in Greece, GRLDaV has also been reported in Italy [
7], Turkey [
8], and Croatia [
9]. The previous evidence of the ability of this virus to damage grapevine crops and to cause losses, along with its emergence in several European countries, point to the need for the control and prevention of its spread. In fact, GRLDaV was included in the European and Mediterranean Plant Protection Organization (EPPO) alert list as a potential phytosanitary risk for the EPPO region in October 2018. Management and control of viral pathogens and prevention of their spread lay on early detection based on specific and reliable diagnostic methods. Quantitative real-time PCR methods have been shown to be powerful tools for viral epidemiological studies and viral detection [
10,
11,
12,
13,
14,
15]. This work aimed to develop a specific real-time PCR detection method that can perform an absolute quantitation of GRLDaV genetic material in both plant material and mealybugs. Such a technique could be applied to epidemiological studies and would provide a powerful tool to address control strategies designed to prevent GRLDaV spread.
3. Discussion
Quantitative real-time PCR detection methods are powerful and reliable diagnostic tools for the control and management of viral diseases. In this study, we report the development of a specific real-time qPCR method that allows the detection and absolute quantitation of GRLDaV, a grapevine virus included in the EPPO alert list as a potential phytosanitary risk for the EPPO region. This technique performs specific and reliable quantitative detection of GRLDaV in both plant material and mealybugs, a key step to preventing the spread of this pathogen in the European and Mediterranean vineyards.
The GRLDaV genomic sequence shows a high degree of variability, related to its geographical distribution, which might compromise its reliable diagnosis. With the aim of designing a detection method able to deal with this variability, viral sequences from all known GRLDaV-affected geographic locations were analyzed. The real-time qPCR method developed in this study has been shown to specifically cover all known GRLDaV diversity by using a unique TaqMan probe in a highly conserved region of the RNase H domain in ORF4. Five primers, three forward primers, and two reverse primers, working in a single multiplex reaction, were designed to amplify a small region containing the probe target sequence as an alternative of highly degenerated primers that could compromise efficient primer concentrations and detection sensitivity. In fact, the technique has been shown to exhibit not only a high degree of specificity towards its target but also a high level of sensitivity in GRLDaV detection. In addition, this method can also be successfully applied for the absolute quantitation of GRLDaV in both plant material and mealybugs.
The GRLDaV detection method here described has been validated evaluating its analytical sensitivity, analytical specificity, selectivity, and repeatability and reproducibility according to the EPPO guidelines.
First, the method showed a high sensitivity in GRLDaV detection using both crude extracts and purified DNA. In fact, the sensitivity of the technique was much higher than the sensitivity reached by two previously reported detection methods based on conventional generic PCR [
16] and SYBR Green real-time PCR [
4]. The comparison between the three techniques showed that the sensitivity of the new method was two orders of magnitude higher with respect to the conventional PCR and five orders of magnitude higher with respect to the SYBR Green real-time PCR, with a detection limit of around 30 copies in naturally infected plant material. Moreover, the real-time qPCR developed in this work was able to test crude extracts with a similar sensitivity to the previously reported real-time method testing purified DNA samples. This result raises the possibility of avoiding nucleic acid extraction steps for rapid testing, although sensitivity is two orders of magnitude higher when purified DNA is tested. In any case, the method reported herein provides increased sensitivity in GRLDaV detection compared to existing diagnostic tools.
In addition, the specificity and selectivity of our method were also validated. The assay turned out to be highly specific according to the results obtained for both inclusivity and exclusivity. All GRLDaV isolates analyzed from different geographic origins covering the actual known viral diversity tested positive by the technique. On the other hand, none of the sanitized grapevine nor GRLDaV-free grapevine plants bearing several grapevine common viruses tested positive in our assay, demonstrating the high level of analytical specificity of the method provided herein. In addition, the selectivity of the assay was also shown by its accurate performance in different cultivars of the host plant.
Finally, the repeatability and reproducibility of the method were also demonstrated. A series of nine technical repeats of seven relatively low titer samples tested with three different thermal cyclers on different days and by different operators was successfully performed. The coefficient of variation observed in the analysis results was lower than 9% for all the samples tested, indicating the absence of significant deviations on the technique performance.
The validated technique was applied for the detection of GRLDaV in mealybugs P. citri and P. viburni. Under our experimental conditions, both mealybug species were able to carry the virus, with their viral titer being successfully quantified by our method. Whether these mealybug species are indeed transmission vectors of this virus remains to be studied. However, the ability of this technique to be applied for those epidemiological studies and used for vector-mediated spread control has been demonstrated.
In conclusion, in this study, we have developed a novel real-time qPCR method for the detection and absolute quantitation of GRLDaV that has been successfully applied for diagnosis in both grapevine plants and mealybugs. The technique has been validated according to EPPO guidelines showing a high sensitivity, specificity, selectivity, and repeatability and reproducibility. This new detection method could contribute to future epidemiological studies and to preventing the spread of this potential pathogen between grapevine plants.
4. Materials and Methods
4.1. Plant Material
A total of 22 GRLDaV infected grapevine samples from different origins and varieties were selected as positive controls for this study: 17 samples from Greece (cv. Roditis, Dafnia, Mavrothiriko, Moschato Samou, Korinthiaki stafida, Romeiko, Lagorthi, Assyrtiko, and Koumariano); 2 samples from Turkey (cv. Yalova incisi) kindly supplied by Dr. Ulubaş Serçe (Niğde Ömer Halisdemir University); 2 samples from Italy (cv. Bombino Nero) kindly supplied by Dr. Minafra (CNR-IPSP); and 1 sample from Croatia (cv. Ljutun) kindly supplied by Dr. Vončina (University of Zagreb). The presence of GRLDaV in all positive samples was confirmed by PCR using two different sets of primers. Badna-R-Up (5′GAAGGAATTGAATCTCCAGCAGCAGG3′, sense) and Badna-R-Do (5′CTCTGCTACACCAAGTGATAGATTGTTGAG3′, antisense) [
4] and Badna-FP (5′ATGCCITTYGGIAARAAYGCICC3′, sense) and Badna-RP (5′CCAYTTRCAIACISCICCCCAICC3′, antisense) [
16]. Plant material from grapevines (cv. Bobal, Tempranillo, Marsaoui, Razzegui, Red Globe, and Crimson seedless) analyzed by high throughput sequencing (HTS), harboring different non targeted viruses, and from Spanish grapevine cultivars (cv. Aledo and Ideal) sanitized by tip culture was used as negative control and for specificity assays. In addition, 65 grapevine samples randomly collected from two Greek grapevine growing areas (Athens and Pieria) were analyzed.
4.2. Crude Extract Preparation and DNA Purification
Leaf tissue from each plant sample was placed in individual plastic bags (Bioreba, Reinach, Switzerland). Extraction buffer (PBS containing 0.2% diethyldithiocarbamate and 2% PVP-10) was added up to a 1:5 ratio (w:v). Homex 6 homogenizer (Bioreba, Reinach, Switzerland) was used for grinding. For crude extract qPCR real-time analysis, 1:50 plant extract dilutions were performed in H2O, centrifuged at 20,000× g for 1 min, heated at 95 °C for 10 min, and placed on ice. DNA was purified from 200 µL of the same plant extracts using a Plant/Fungi DNA isolation kit (Norgen Biotek Corporation, Thorold, ON, Canada) following the manufacturer’s instructions. Extracted DNA was quantified with a DeNovix DS-11 spectrophotometer (DeNovix Inc., Wilmington, DE, USA) to determine the DNA concentrations and stored at −80 °C until subsequent analysis.
Total DNA was extracted from mealybugs using a Plant/Fungi Total DNA Purification Kit (Norgen Biotek Corporation, Thorold, ON, Canada) with slight modifications. Briefly, 400 μL of PBS 1X buffer was added to tubes containing 1, 2, 4, or 5 mealybugs. The suspension was vortexed for 5 min in the presence of 212–300 μm glass beads (Sigma-Aldrich, St. Louis, MO, USA). After centrifugation at 10,000× g for 5 min, 200 µL of the clarified supernatant was transferred to a fresh tube, and the extraction procedure was completed following the manufacturer’s instructions.
4.3. Amplification and Sequencing of a Partial GRLDaV RNaseH/ORF4 Genomic Region
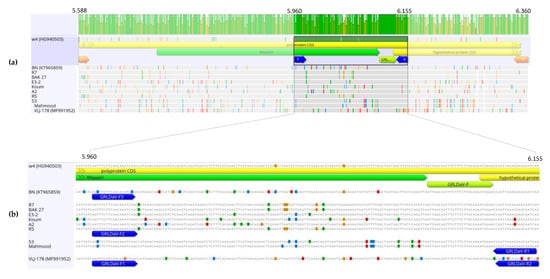
Alignment of the three GRLDaV full genomes available in the databases [
17] corresponding to isolates W4, BN, and VLJ-178 from Greece, Italy, and Croatia, respectively (accession numbers HG940503, KT965859, and MF991952) was performed using ClustalW implemented in Geneious software 10.0.7 (Biomatters Ltd., Auckland, New Zealand). Based on this alignment, primers targeting a 775 nt in the GRLDaV genome including part of the RNaseH sequence (ORF3) and part of the ORF4 region, GRLDaV-RNaseH-F (5′AGCCCACTCTATGCYAARACAAG3′, sense), and GRLDaV-RNaseH-R (5′TGCTTTCTTTGCTGACTCAGCTTC3′, antisense) were designed manually. Primers and probe melting temperatures and secondary structures were analyzed using the online OligoAnalyzer Tool (Integrated DNA Technologies Inc., Coralville, IA, USA). A 775 bp fragment was amplified from nine selected GRLDaV positive samples from different origins: sample R5 (Crete, Greece); sample R7 (Crete, Greece); sample BAK-27 (Nemea, Peloponese, Greece); sample Koum (Tinos island, Greece); sample D2-1 (Grapevine Institute, Athens, Greece); sample E3-2 (Grapevine Institute, Athens, Greece); sample VLJ-178 (Kaštela, Croatia); sample 53 (Adana province, Turkey) and sample Mahmood (Adana Province, Turkey). PCR amplification was performed using Platinum Taq DNA polymerase (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions. The reaction mixture contained 0.5 µM of each primer, 0.75 µL of KB Extender, and 40 ng of DNA in a volume of 3 µL, in a total reaction volume of 25 µL. PCR protocol consisted of a first step at 94 °C for 2 min, followed by 35 cycles of amplification (30 s at 94 °C, 30 s at 55 °C, and 48 s at 72 °C). All PCR amplicons were cleaned using mi-PCR Purification Kit (Metabion International AG, Martinsried, Germany), and Sanger sequenced in both directions.
4.4. Primers and Probe Design
All amplified RNaseH/ORF4 sequences, as well as the corresponding genomic region of the GRLDaV full-length sequences available in the databases (HG940503, KT965859, and MF991952), were aligned using ClustalW implemented in MEGA X software [
18]. Based on the alignment, a set of five primers and a probe were designed for the real-time PCR assay. Three forward primers and two reverse primers were designed to cover all the variability detected in the selected region, yielding an amplicon of 196 bp: GRLDaV-F1 (5′AGTTTCTTCAACAAGTCAGC3′, sense); GRLDaV-F2 (5′AGTTTCTTCAACAAATCAGC3′, sense); GRLDaV-F3 (5′AGCTTCTTCAACAAATCCG3′, sense); GRLDaV-R1 (5′TGATTGCTCRTTTAACTGG 3′, antisense); and GRLDaV-R2 (5′ GACTGTTCCTTCAGCTG 3′, antisense). A TaqMan probe, GRLDaV-P (5′-6-FAM-ATTAACAGTTTCTTTCTTACAGAATGGAA-BHQ1-ZNA4-3′) showed specificity towards all the sequences used in the study.
4.5. TaqMan Quantitative Real-Time PCR
TaqMan qPCR was performed in three different real-time thermal cyclers: StepOne Plus thermal cycler (Applied Biosystems, Foster City, CA, USA); QuantStudio 3 real-time PCR system (Applied Biosystems, Foster City, CA, USA), and LightCycler 480 (Roche, Basel, Switzerland), using a Premix Ex Taq master mix for probe-based real-time PCR (Takara Bio Inc., Kusatsu, Japan) according to the manufacturer’s instructions. Five microliters containing 70 ng of DNA or 5 µL of crude extract were used as a template. The reaction mixture contained 250 nM of the probe and 0.35 µM of each of the primers in a total reaction volume of 20 µL. For reactions performed in the Applied Biosystems instruments, 0.4 µL of the ROX Reference Dye (50X) was added as a passive reference dye. PCR reaction consisted of an initial denaturation step at 95 °C for 2 min, followed by 45 cycles of amplification (15 s at 95 °C and 1 min at 60 °C). Data acquisition and analysis were performed using the corresponding software for each thermal cycler. The default threshold set by the machines was slightly adjusted above the noise to the linear part of the growth curve at its narrowest point, according to the manufacturer.
4.6. Generation of Real-Time qPCR Standard Curves
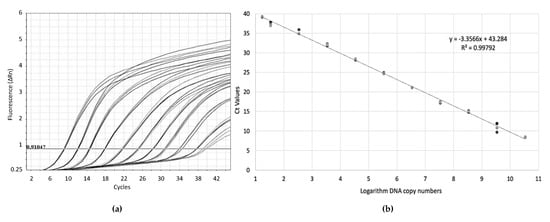
For the generation of real-time qPCR standard curves, the RNaseH fragment targeted by the qPCR was amplified by conventional PCR using primers GRLDaV-F2 and GRLDaV-R1. The 196 bp PCR product was purified using a PCR Purification Kit (Metabion International AG, Martinsried, Germany) following the manufacturer’s instructions, inserted in the pGEM-T Easy vector (Promega Corporation, Madison, OH, USA), and cloned into
Escherichia coli XL1-Blue. Transformant colonies were selected by ampicillin resistance. Plasmid extraction was performed using the mi-Plasmid Miniprep Kit (Metabion International AG, Martinsried, Germany) following the manufacturer’s instructions and quantified with a DeNovix DS-11 spectrophotometer (DeNovix Inc., Wilmington, DE, USA). Plasmid DNA was quantified in picomoles applying the following mathematical formula: pmol of dsDNA = (μg of dsDNA × 10
6)/(660 × Nbp) considering the average molecular weight of a pair of nucleotides (660 Da) and the number of base pairs of the plasmid carrying the GRLDaV partial genomic RNaseH sequence (Nbp). The Avogadro constant [
19] was used to estimate the number of plasmid molecules (6.023 × 10
23 molecules/mol). Three replicates of serial dilutions from 3 × 10
10 to 2 plasmid copies were prepared and used to generate the standard curve. The amplification efficiency of the calibration curve was calculated by the slope, according to the mathematical formula: amplification efficiency = [10
(−1/slope)]−1 [
20].
4.7. Validation of the Real-Time qPCR Method
The real-time qPCR method developed in this study was validated according to the EPPO guidelines [
21]. Thus, analytical sensitivity (maximum dilution of DNA detected), analytical specificity (inclusivity, detection of variants/strains covering genetic diversity and different geographic origin; exclusivity, negative detection of relevant non-targets that might be present in the matrix), selectivity (evaluation of test performance using different cultivars), and repeatability and reproducibility (evaluation of the test performance consistency on different replicates by different operators and with different equipment) were evaluated.
4.7.1. Evaluation of the Sensitivity
Analytical sensitivity of the real-time PCR developed in this study was evaluated using crude extracts or purified DNA from GRLDaV naturally infected plant material. The crude extracts were tested at 1:50 dilution in distilled H
2O. DNA was purified from GRLDaV infected plant extract and from ten-fold serial dilutions (from 10
−1 to 10
−8) of the infected plant extract in healthy plant extract, to avoid dilution of putative PCR inhibitors present in the matrix. Absolute quantitation was performed, generating standards curves as described above, and viral titer, the number of viral targets per volume, was determined. Three replicates of each sample were tested. All samples were tested by two previously described GRLDaV detection methods [
4,
16] for sensitivity comparison.
4.7.2. Evaluation of the Specificity
The inclusivity and selectivity of the developed detection method were evaluated by testing GRLDaV isolates from different geographic origins showing genetic diversity and infecting different cultivars.
Exclusivity was evaluated by testing eight grapevine samples infected by several viruses commonly present in the host. The virome of these samples, not infected by GRLDaV, was determined by high throughput sequencing (HTS), as described previously [
22]. Briefly, total RNA was extracted from leaves and sequenced after ribo-depletion using RNAseq TrueSeq Illumina technology (150 nt pair-end reads). Data analysis was performed by CLC Genomics Workbench 10.1.1 (QIAGEN, Hilden, Germany). Raw data were subjected to quality control, trimming, and host genome subtraction steps, and the resulting reads used to assemble de novo contigs (minimal contig length 200 nt). The contigs similarity to viral sequences was analyzed by BLASTn and BLASTx with a cut-off e-value of 10
−4.
4.7.3. Evaluation of the Repeatability and Reproducibility
For evaluation of the repeatability and reproducibility of the real-time qPCR, seven surveyed samples that tested positive for GRLDaV with the lowest viral titers were selected. Both crude extract and purified DNA of the selected samples were tested in nine technical repeats using three different thermal cyclers, QuantStudio 3 real-time PCR system Plus (Applied Biosystems, Foster City, CA, USA), StepOne Plus (Applied Biosystems, Foster City, CA, USA), and LightCycler 480 (Roche). For each of the instruments, 3 independent repeats were performed, carried out on different days, and by different operators. Technical repeats included 3 replicates for each sample. Mean Ct values and coefficients of variation were calculated.
4.8. Quantitative Detection of GRLDaV in Mealybugs
Colonies of the
P. citri and
P. viburni mealybugs were kindly provided by Dr. Beitia (IVIA). Adult mealybugs were originally collected from persimmon fruits (
Diospyros kaki L.) located in a persimmon growing area in Algemesí (Ribera del Xúquer, Valencia, Spain). Mealybug colonies were maintained in lemon fruits at 26 °C for 60 days. During this period, the absence of GRLDaV in the mealybugs was confirmed twice, every 30 days, by testing 5 pools of 5 mealybugs by both the conventional and the real-time GRLDaV PCR detection methods previously described [
4,
16] and by the real-time qPCR method developed in this study. GRLDaV-free mealybugs were transferred to both GRLDaV infected and healthy grapevine leaves. Acquisition was performed at 28 °C, 60% humidity and a 12/12 h light cycle for 48 h in a plant growth chamber (Sanyo MLR-350H). After this time, pools of 2 and 4 mealybugs, as well as individual insects, were analyzed by the real-time qPCR developed in this study. These experiments were performed in nine technical repeats.